15 Which of the Following Systems Has the Highest Entropy
By dividing by 27315. Which of the following has the highest entropy S.

Review Of High Entropy Ceramics Design Synthesis Structure And Properties Journal Of Materials Chemistry A Rsc Publishing Doi 10 1039 C9ta05698j
Water vapor What is the term that describes the tendency in nature for systems to become less ordered or.
. Solids have the fewest microstates and thus the lowest entropy. I Pure alumina Al2O3 s OR ruby ii one mole of N2 g at 1 bar pressure OR one mole of N2 g at 10 bar pressure both at 298K iii dry ice solid CO2 at -78 degrees C OR CO2 g at 0 degrees C. ASur is less than 0 and its magnitude is less than ASsys b.
When an ideal gas expands adiabatically and reversibly from 150 L to 300 L its temperature decreases. CH2 HC CH2 HC Ha During a spontancous chemical reaction it is found that ASsys is less than 0. Which of the following systems has the highest entropy.
Which substance listed below would be expected to have the greatest entropy value S. History 16072019 1530. Arrange the following substances in the order of increasing entropy at 25CHF g NaF s SiF4 g SiH4 g Al slowest highest.
By adding 27315 b. All are at the same temperature T2. Just so which element has the highest entropy.
This problem has been solved. That depends on what you mean by cold system. Salt dissolved in a container of water.
1 mol of HCN c. 10mL of water at 50 degrees c. If we look at the three states of matter.
Which of the following probably has the highest entropy at 298 K. 2 mol of HCN. Which one of the following systems has the highest entropy.
The substance in the gaseous state has the highest entropy compared to solid and liquid state. CH 3CH 2OH e. Chemistry 22062019 0720.
Which of the following systems has the greatest root-mean-square RMS velocity of the atoms. For liquid state still the particles are moving but less freely than the gas particles and more than the solid particles. For which of the following processes does the entropy of the system decrease.
Indicate which of the following has the lowest standard molar entropy S. 1 Show answers Another question on Chemistry. If a system is left to change spontaneously in what state will it end.
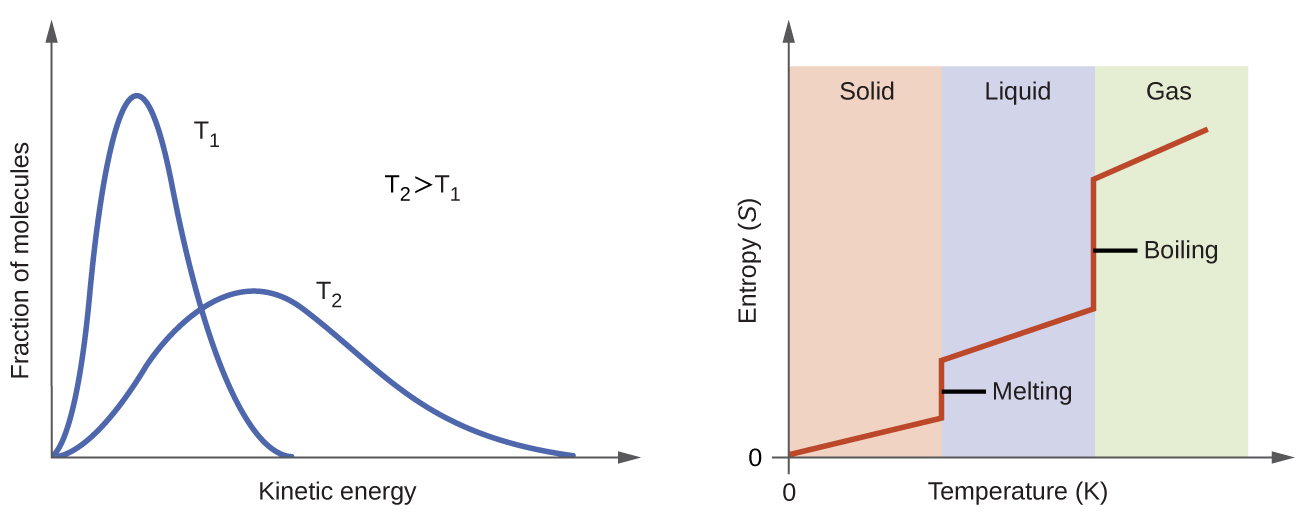
S_gasS_liquidS_solid Entropy by definition is the degree of randomness in a system. For processes involving an increase in the number of microstates W f W i the entropy of the system increases ΔS 0. Hence here the gas is only hydrogen and it has the highest entropy.
Which of the following has the highest entropy all at 650. Which of the following has the highest entropy S. See the answer See the answer See the answer done loading.
All of these lead to a positive change in entropy of the system as they are. For the second question the system with the lowest entropy is sugar crystals in 95 degrees centigrade cup of coffee. Entropy is a measure of chaos or disorder in a system.
Which of the following has the highest standard molar entropy at 29815 K A CF 4 from CHEM 123 at University of Waterloo. When a substance is a gas it has many more microstates and thus have the highest entropy. Entropy is a measure of disorder.
This means that a. None - all of these have the same entropy b. 10 mL of water at 100 C d.
4210 to the negative 2 power divided by 210 to the 2nd power. 10mL of water at 10 degrees c. For the first one the system with the highest entropy is a cup of water at 0 degress centigrade.
By subtracting 27315 c. Liquids have more microstates since the molecules can translate and thus have a higher entropy. Therefore hydrogen has the highest Entropy.
If the enthalpy change for the transition of liquid water to steam is 3 0 K J m o l 1 at 2 7 o C the entropy change for the process would be in J m o l 1 K 1. This is the best answer based on feedback and ratings. Which substance has the higher entropy in the following options.
Which of the following systems has the highest entropy. Rank the five processes according to the change in entropy of the gas least to greatest. 2 Get Other questions on the subject.
10 mL of water at 10 C b. 10mL of water at 100 degrees c. HC Ha Ho Hz нс H2 Ha C c.
All have the same entropy because all are water. Which of the following has the highest entropy when produced in a reaction. Increase decrease or remain constant.
05 g of HCN b. 10 mL of water at 100C 10 mL of water at 10C 10 mL of water at 50C. Conversely processes that reduce the number of microstates W f W i yield a decrease in system entropy ΔS 0.
Which of the following has the highest entropy S. 2 kg of HCN d. This molecular-scale interpretation of entropy provides a link to the probability that a process will occur as illustrated in the next paragraphs.
Which of the following systems has the lowest entropy. 88 which one of the following systems has the highest. CH CH CH CH нс HC Ha Hz CHs CHs CHa Ha Ha H2 e.
Hydrogen is in the gaseous state. The mass of carbon dioxide is 288 g The temperature of the gas is 22oC 22oC27315 29515 K. Which of the following systems has the highest entropy.
A cup of ice at 0 degrees c a cup of water at 50 degrees c a cup of water at 0 degrees c a cup of ice at 15 degrees c. An ideal gas is to taken reversibly from state i at temperature T1 to any of the other states labeled I II III IV and V on the p-V diagram below. Entropy in any system can do one of three things.
It will be maximum in gases followed by liquids and least in solids. Solid Liquid and Gas we can see that the gas particles move freely and therefore the degree of randomness is the highest. All have the same entropy because all are water.
Beside above which state of matter is likely to. If the system is closed then entropy will only ever increase. Assume all substances are in the gas phase at the same temperature.
10 mL of water at 50 C c. Indicate which of the following has the highest entropy at 298 K. Which of the following has the highest entropy Water Ice Water solution or Water vapor.
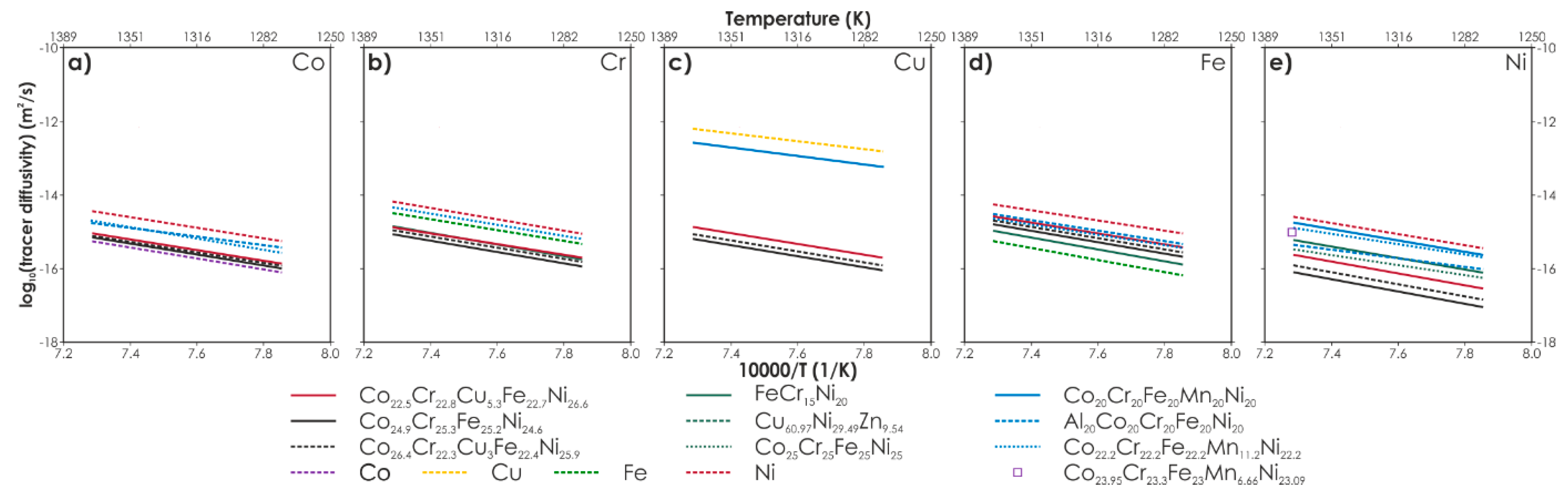
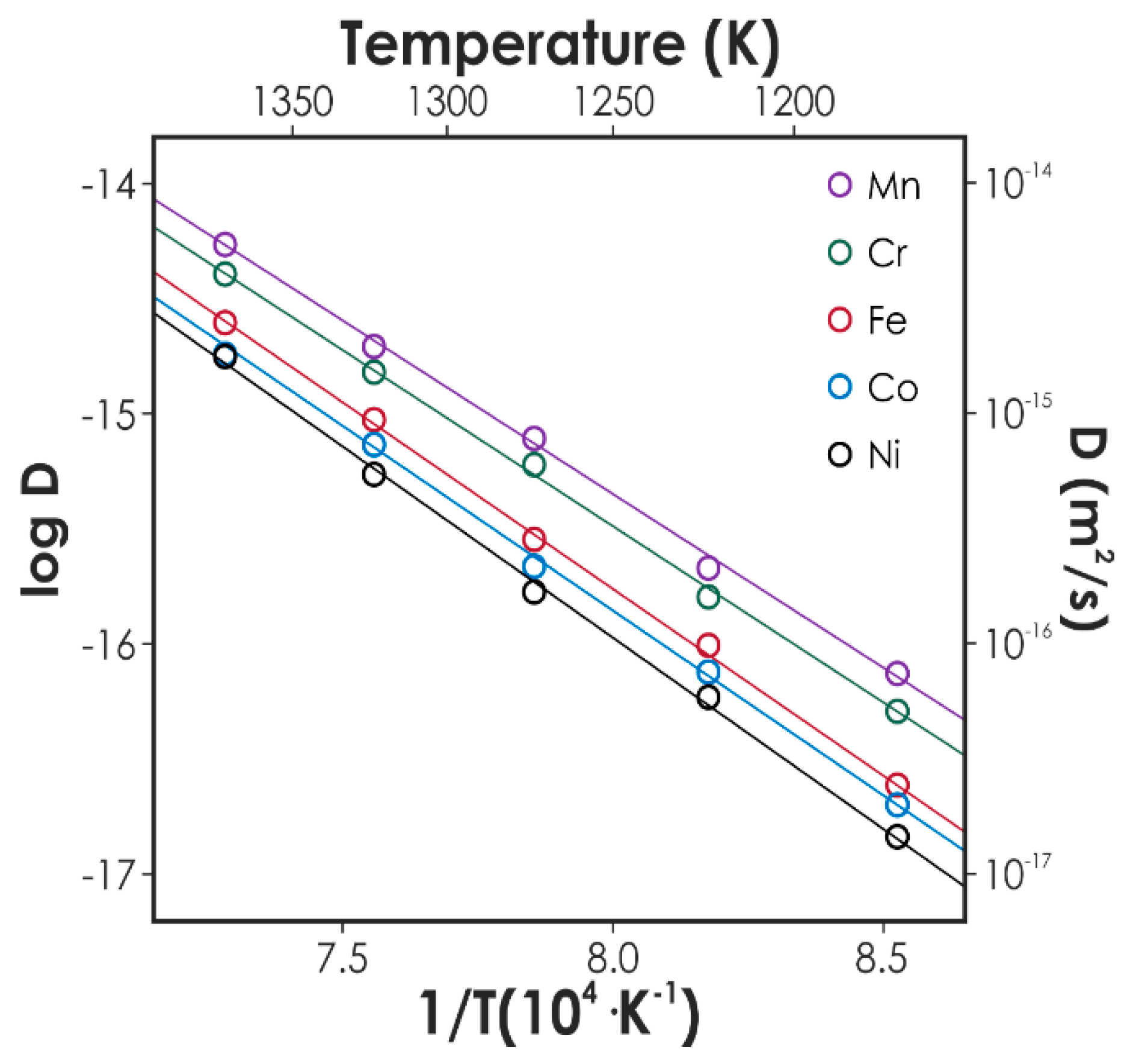
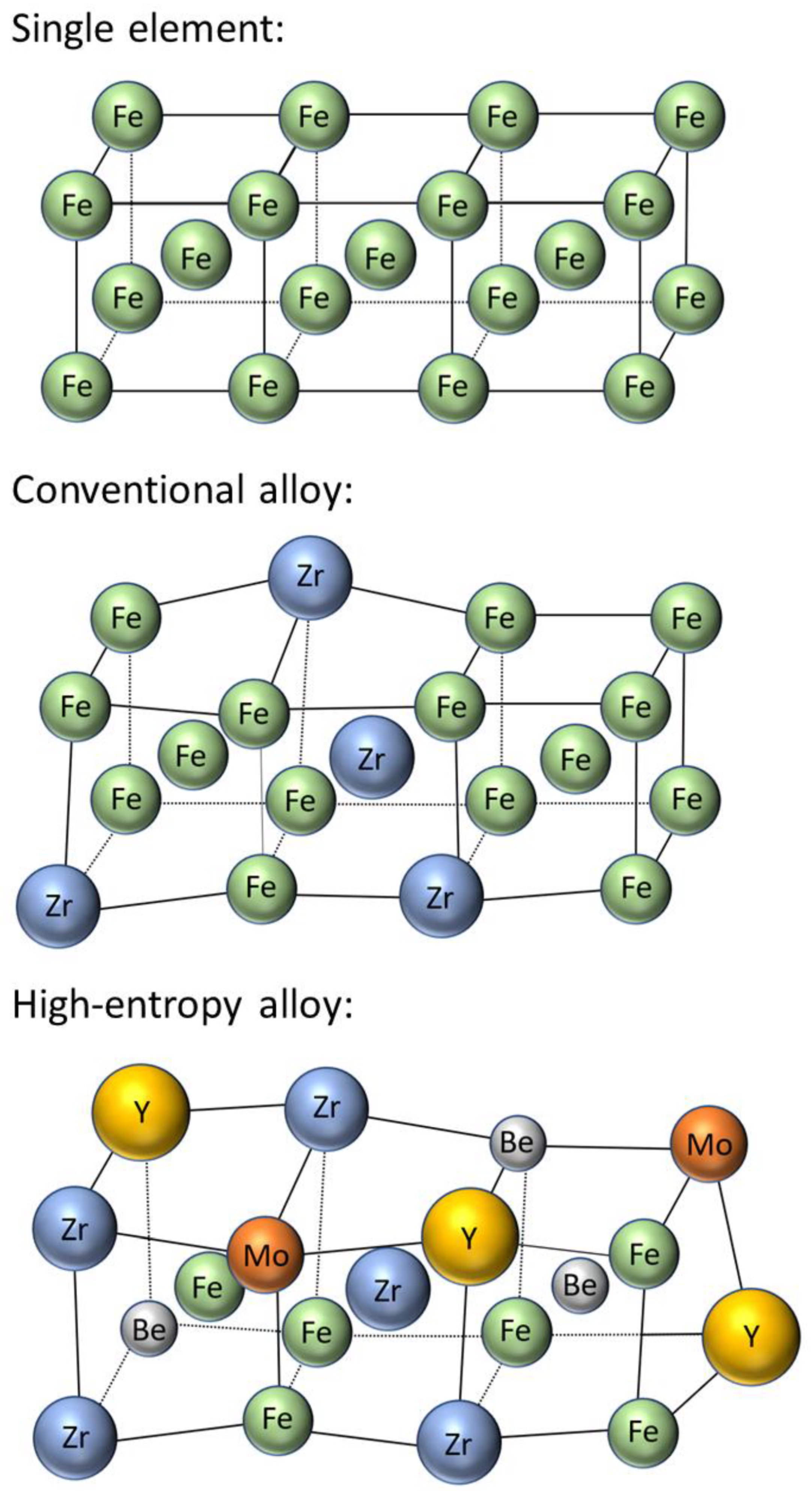
Metals Free Full Text State Of The Art Diffusion Studies In The High Entropy Alloys Html
Metals Free Full Text A Review Of The Serrated Flow Phenomenon And Its Role In The Deformation Behavior Of High Entropy Alloys Html
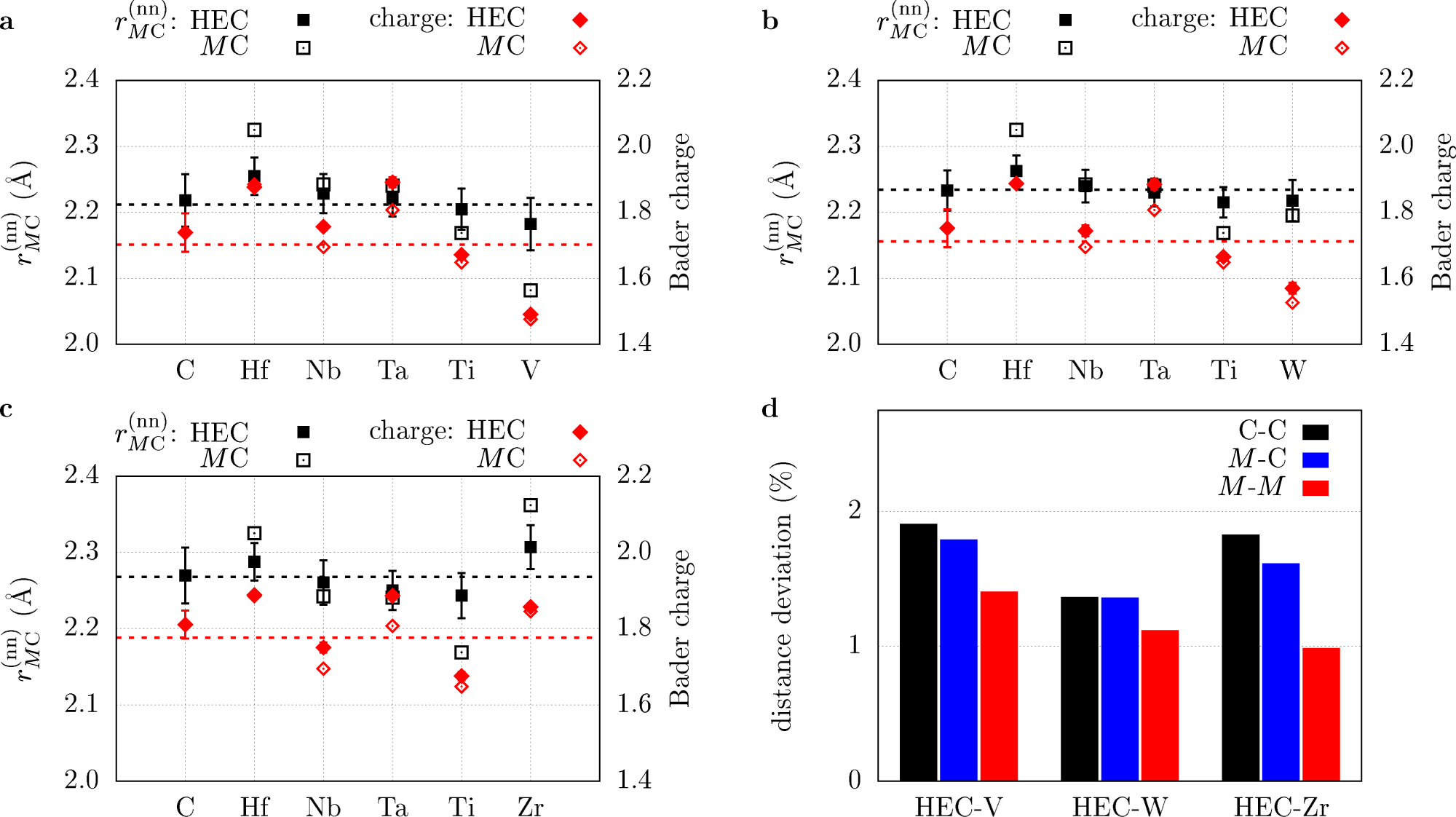
Settling The Matter Of The Role Of Vibrations In The Stability Of High Entropy Carbides Nature Communications
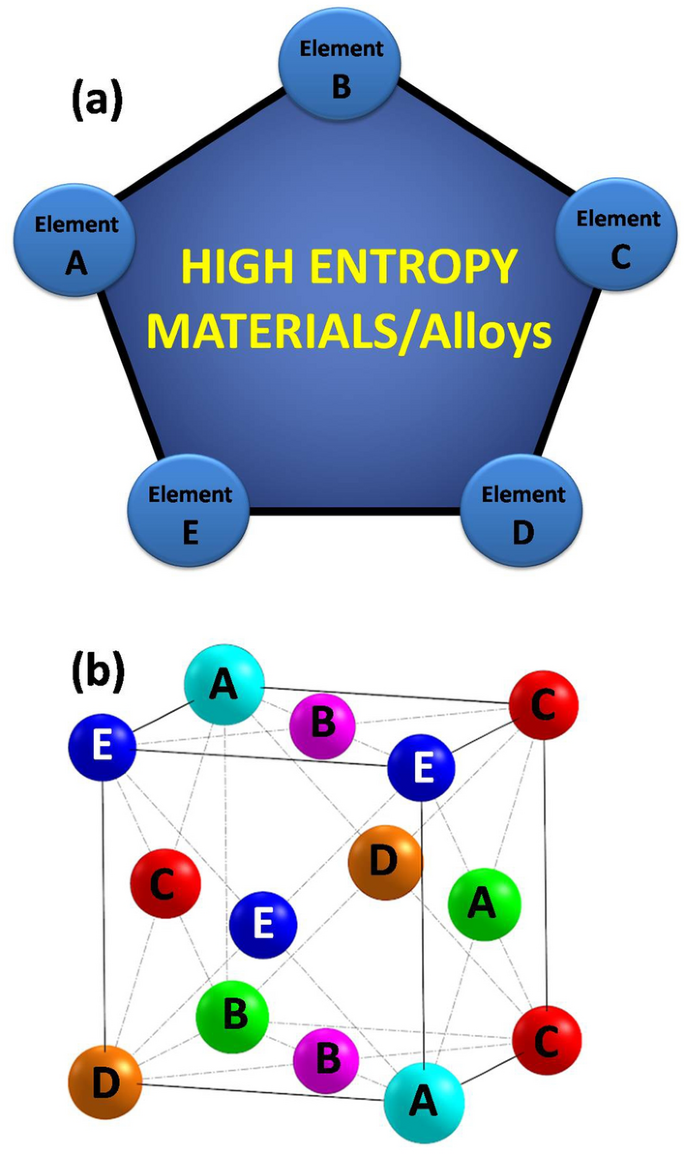
High Entropy Alloys For Solid Hydrogen Storage Potentials And Prospects Springerlink

Thermochemistry And Thermodynamics Ppt Download
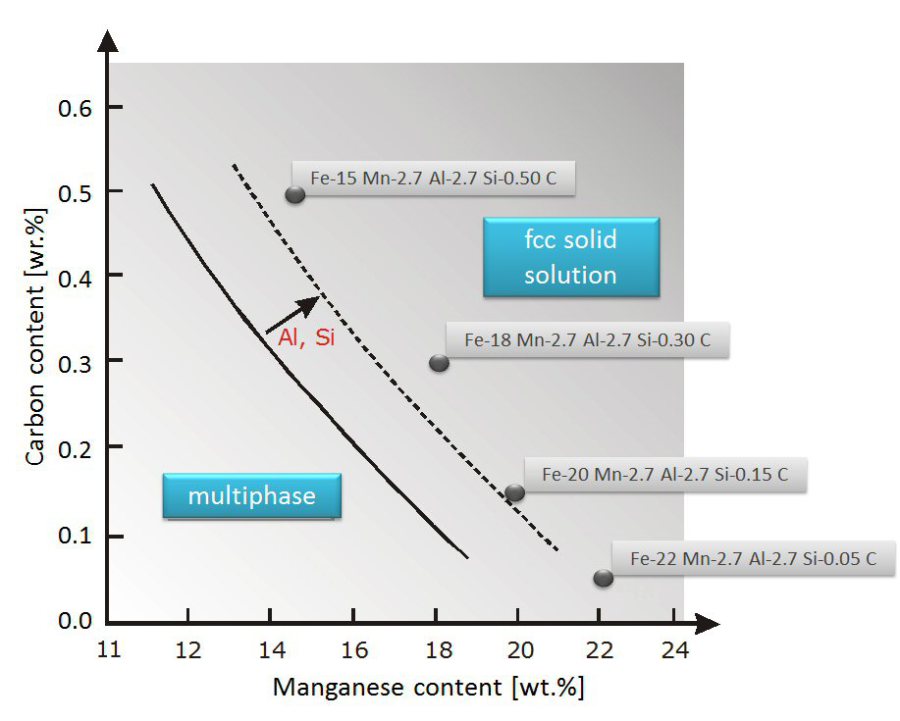
Metallurgical Materials Science And Alloy Design High Entropy Steels

Metals Free Full Text State Of The Art Diffusion Studies In The High Entropy Alloys Html

Which State Of Matter Has The Highest Entropy Study Com
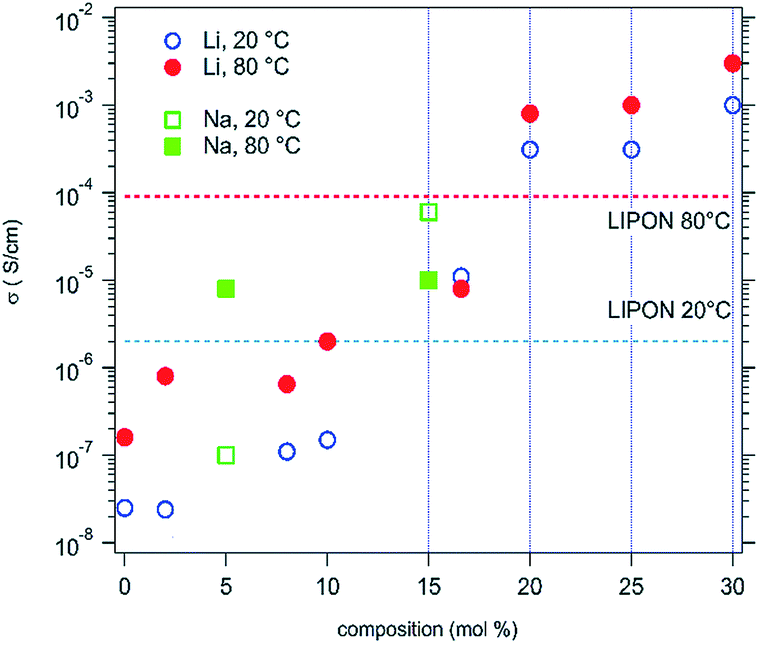
Review Of High Entropy Ceramics Design Synthesis Structure And Properties Journal Of Materials Chemistry A Rsc Publishing Doi 10 1039 C9ta05698j

High Entropy Alloys Emerging Materials For Advanced Functional Applications Journal Of Materials Chemistry A Rsc Publishing Doi 10 1039 D0ta09601f

Pdf Recent Advances Of High Entropy Alloys For Aerospace Applications Literature Review

Recent Progress Of High Entropy Materials For Energy Storage And Conversion Journal Of Materials Chemistry A Rsc Publishing Doi 10 1039 D0ta09578h

What Is Entropy A Measurement Of The Degree Of Randomness Of Energy In A System The Lower The Entropy The More Ordered And Less Examples Gallon Of Gas Pre Science




Comments
Post a Comment